This is a project to create a poster showing all 118 chemical elements in a continuous spiral, rather than the ubiquitous periodic table. I did not originate the idea of using a spiral to show the relationships between the elements, but this design is my work. In one version, I've even added an entire (still hypothetical) eighth period, which includes space for potential additions from element 119 all the way through element 172. The most recent version leaves out Period 8 for the sake of simplicity, and notes that 2019 was the 150th anniversary of the original periodic table devised by Russian chemist Dmitri Mendeleev.

This is a way of representing the chemical elements that I personally find more intuitive than its close relative, the periodic table. Think of the traditionally-oriented table as a very long sentence on a page in a book, where line breaks occur at the right side of the page out of necessity, with sentences (and sometimes even hyphenated words) simply continuing one line down, back on the left side of the page. The reader understands that the line breaks are immaterial to the status of the sentence being read, and that the sentence continues until she comes to the full stop punctuation (commonly referred to as a "period," but no relation to the chemical periods that are our subject here).
Now imagine cutting out all of the individual lines of text from the book's page, and taping them together so that the entire sentence runs from the first to last word, without interruption even for line breaks.* Then wind that single uninterrupted sentence into a spiral so that you could fit it on a single page; I have simply done the same thing with the periodic table of the elements. I've also included a version of the traditionally-oriented periodic table with all of the colors, typography, periods, groups, atomic symbols and atomic numbers represented similarly, to aid in orienting a viewer already acquainted with the table to this alternative organization of the same information.
In the spiral, the groups appear as stacks of elements radiating outward from the center of one of the diagram’s ring structures. The main ring represents the s-block and p-block sections that are at the left- and right-most edges of the standard table. The ring at lower left is the d-block elements, which occupy the middle section of the traditional table, and are commonly referred to as the transition metals. The ring at lower right corresponds to the f-block Lanthanide and Actinide series, sometimes called rare earth metals. These series are almost always pulled out of the standard table to prevent its aspect ratio straying too far from the shape of a textbook page, though in the version of the tabular format included here for reference, that is not the case, and all the rare earth metals discovered to date can be seen in their correct place in the sequence. Element 118, Oganasson is the heaviest element yet synthesized or discovered, and the last one in Period 7.
Acknowledgements: The fundamental insight leading to this general scheme for organizing the elements is usually credited to Dmitri Mendeleev, who first produced his table in 1871 (and is the eponym for element 101). Mendeleev’s table had fewer groups (columns), as he combined the elements with like valences into single groups, a relationship that is emphasized here with coloring, and in the traditional table with similar group names (i.e. I A and I B, a.k.a. 1 and 11, or VII B and VII A, a.k.a. 7 and 17). The person principally responsible for the layout of the most-recognizable version of the periodic table, particularly the convention of placing the Lanthanide and Actinide series below the main body of the table, was long-time UC Berkeley professor (and eponym for Element 106) Glenn Seaborg. There have been many alternative versions of the table, from slight variations to radical departures, but the earlier spirals that most influenced this one include a colorful image by John Drury Clark, published in the May 16, 1949 issue of Life magazine; Theodor Benfey's table, which was included by Seaborg in an article he wrote for the June, 1964 issue of Chemistry; and an oddly silicon-centric spiral from 1974 by J. F. Hyde, known (not necessarily entirely fondly) as the "father of the augmented breast" for his pioneering work on industrial applications for silicone at Dow Corning in the mid-twentieth century. I’d also like to thank my father, another long-time UC Berkeley professor, for his feedback on this project.

This animation is intended to show the way the spiral corresponds to the traditional periodic table. It does this by lighting up each element on both versions simultaneously.
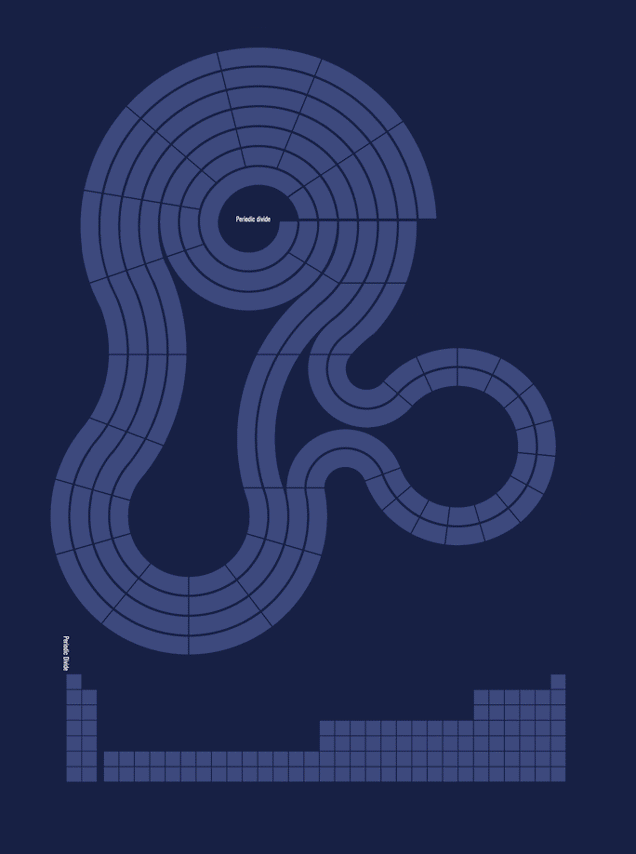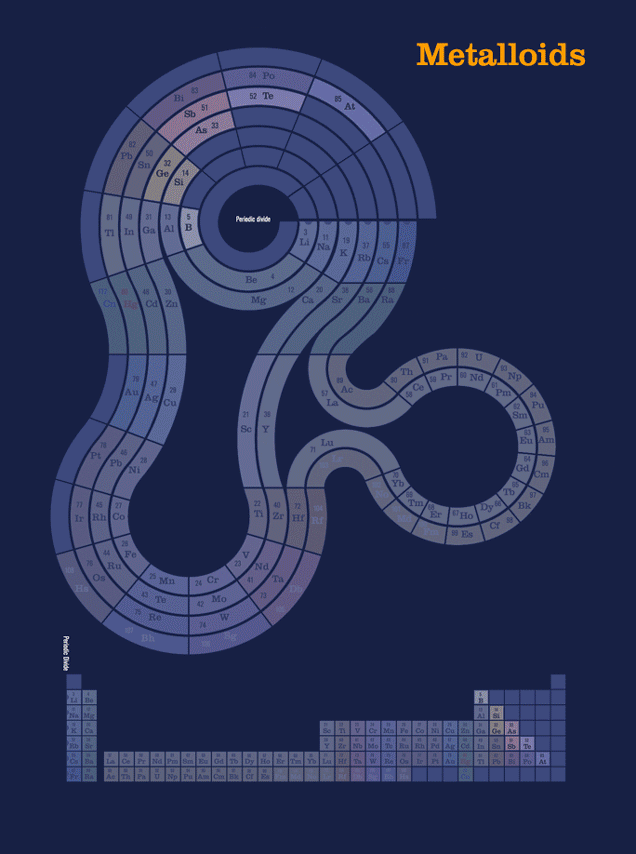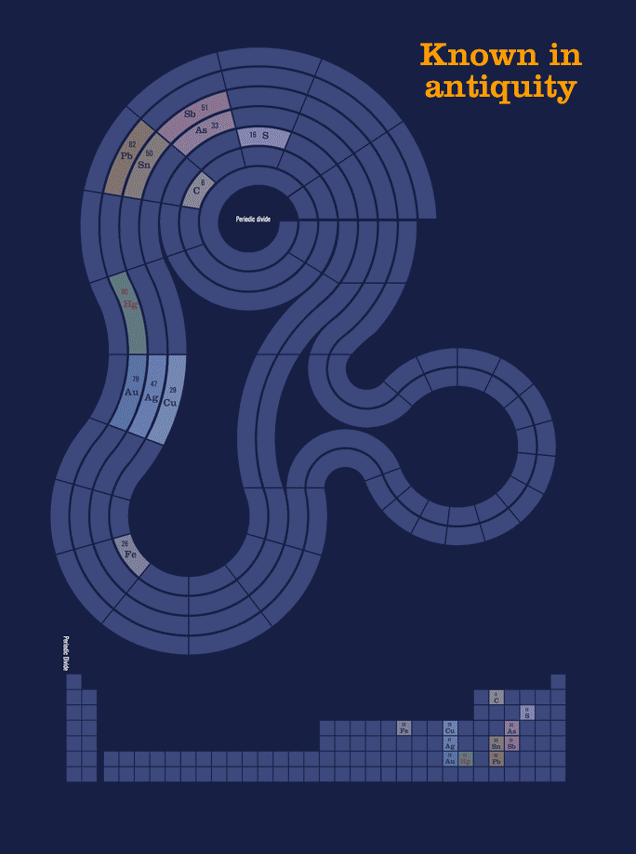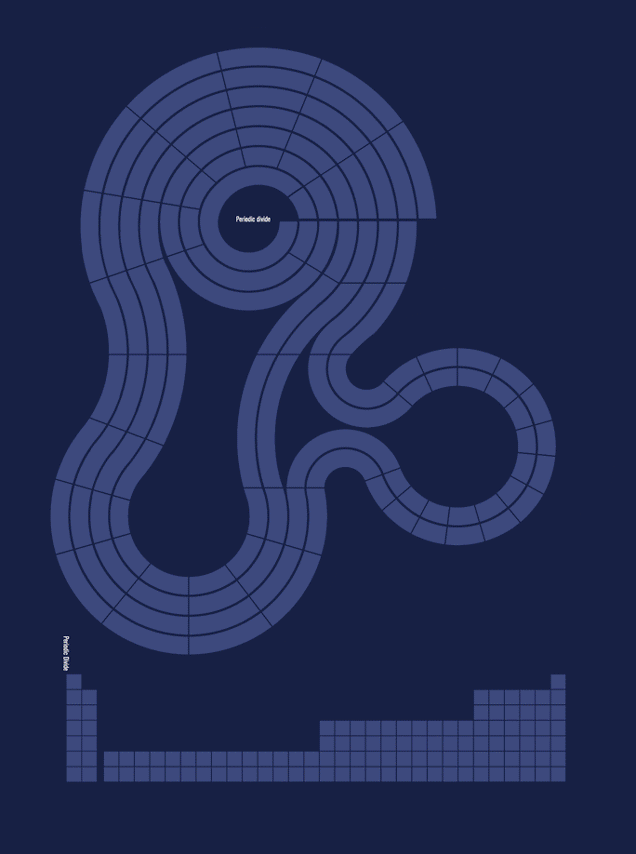
Another animation, this one showing two completely different way of grouping the elements. The first, metalicity, is closely related to each element's position, from left-to-right, as it appears on the traditional periodic table, with trend moving to less metallic as you move rightward.* The second has almost nothing to do wiith chemical properties, but groups the elements by the era in which humans became aware of them (in the case of most of the naturally occurring elements), or synthesized them in laboratories.
*There are other, similar trends not represented here, such as electronegativity, and atomic size, which is somewhat counter-intuitive insofar as the size of an atom of a given element generally gets smaller as you move right within a single period of the table even though you are also adding mass and quantity of subatomic particles. The reason is that the larger number of protons in the nucleus, which of course makes up only a tiny fraction of the atom's size, exert a greater electromagnetic pull on the electrons, whose "orbits" actually define the atom's effective radius, thereby making that radius smaller. However, there is typically a significant jump up in size at the start a new period, due to a new electron shell beginning to be filled.

Older version of the poster

Detail of the older version






